

A USF Spinout Bringing Proven Rehabilitation Technology Home
First truly portable device replicating clinical-grade neuroplasticity effects for over-ground walking. Extends rehabilitation beyond clinic sessions with daily at-home practice. Developed at University of South Florida's (USF) REED Lab. Currently in regulatory pathway development.
Stroke is the leading cause of long-term disability in the United States. Most survivors struggle with persistent gait deficits that limit independence and quality of life. Current solutions are expensive, clinic-bound, and insufficient for the daily practice needed to drive neuroplastic recovery.
Clinical therapy limited to 2-3 sessions per week. Insufficient for optimal motor learning. Transportation, scheduling, and cost create additional barriers to consistent rehabilitation.
Advanced robotic systems are prohibitively expensive. Split-belt treadmills require dedicated clinic space. Only wealthy patients and large institutions can access gold-standard rehabilitation technology. Affordable home solutions are needed to democratize access.
Home exercise programs show poor adherence. Conventional assistive devices don't provide therapeutic stimulus. No affordable solution bridges the gap between clinic-based therapy and home practice.
evergait™ Go is the first truly portable device that replicates clinical-grade neuroplasticity effects for over-ground walking. Passive mechanical design requires no power. Clinical-grade therapeutic benefits at a fraction of traditional clinic-based solutions.
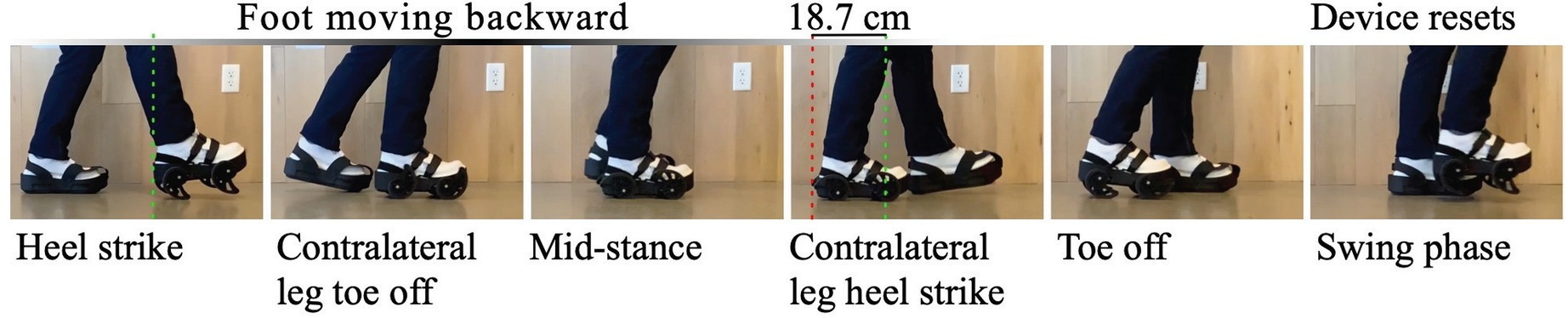
Device creates controlled displacement during gait cycle. From heel strike through swing phase, the mechanism induces therapeutic asymmetry that triggers bilateral neuroplastic adaptation.
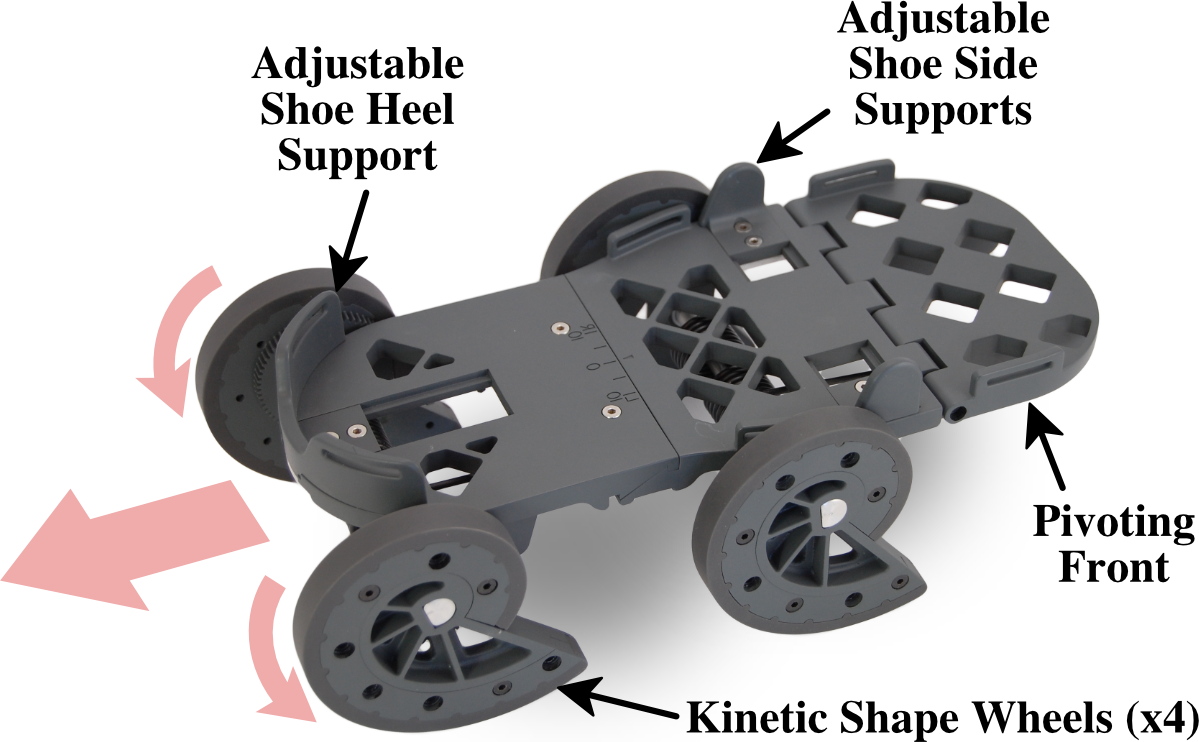
Adjustable heel support and side supports with pivoting front mechanism. Four kinetic shape wheels convert user movement into controlled gait displacement. Mechanical design requires no batteries or power source.
First device for home & community use
NIH-funded with peer-reviewed publications
Exclusive license from USF
Manufacturing partners identified
Regulatory route defined
evergait™ technology is protected by patented innovations licensed from the University of South Florida. Our IP portfolio includes granted patents and additional applications covering core device functionality, manufacturing methods, and smart monitoring systems. This intellectual property represents extensive research and development at USF's REED Lab, supported by NIH and NSF funding.
After extensive research and clinical validation at USF, evergait™ technology is ready for commercialization. With proven clinical efficacy, strong intellectual property protection, and experienced leadership, we're positioned to transform stroke rehabilitation at scale.
• Extensive research foundation: Multi-million dollar federal research support (NIH/NSF)
• Clinical validation: Multiple peer-reviewed publications demonstrating sustained therapeutic effects
• Protected IP portfolio: Patented innovations licensed from USF
• Real-world evidence: Demonstrated patient impact across multiple clinical studies
• De-risked technology: Core feasibility and efficacy already established
• World-class inventor: Dr. Kyle Reed brings deep technical expertise and long-term commitment to product development
• Proven commercial execution: Diana Shapiro's track record includes successful medical device and AI diagnostics companies
• Regulatory expertise: Working with experienced FDA counsel to navigate pathway efficiently
• Strategic focus: Building sustainable value through clinical evidence and market traction
• Aligned incentives: Team committed to long-term success, not quick exits
• Growing need: 7 million US stroke survivors with limited home therapy options
• Reimbursement evolution: Increasing coverage for home-based rehabilitation technologies
• Technology readiness: Manufacturing and quality systems approaching deployment stage
• Strategic partnerships: Building relationships with healthcare providers and medical device leaders
• Capital efficiency: Non-dilutive funding pathway supports sustainable growth
evergait™ combines rigorous scientific validation with experienced commercial leadership to deliver transformative rehabilitation technology. We're focused on generating clinical evidence, achieving regulatory milestones, and building strategic partnerships that create long-term value for patients, healthcare providers, and stakeholders.
Extensive research program at USF's REED Lab, supported by NIH funding. Multiple peer-reviewed publications in leading journals. Consistent efficacy demonstrated across clinical trials with stroke survivors.
53-year-old stroke survivor participating in IRB-approved research study at USF REED Lab
Participant provided informed consent for research documentation. Video from IRB-approved clinical study.
Research Context: This documentation is from an investigational study. The evergait™ Go device is not cleared or approved by FDA for commercial use. Results shown are from controlled research settings and individual outcomes may vary.
Results below are from IRB-approved investigational studies of an investigational device. Findings do not constitute FDA approval or clearance. Clinical outcomes obtained under controlled research conditions with IRB oversight and informed consent. Individual results may vary. Device safety and efficacy profile established under investigational conditions only.
Complete research documentation: USF REED Lab evergait Research Page →
Clear pathway from clinical validation to market deployment. Strategic approach balancing regulatory efficiency with sustainable reimbursement development.
✓ 15 years of NIH/NSF-funded research validation - $2M+ federal investment
✓ Peer-reviewed clinical efficacy demonstrated - Published results in leading journals
✓ Patent portfolio secured with exclusive licensing - Core utility patent granted, additional IP filed
✓ ISO 13485-certified manufacturing capability identified - Scalable production partnership
✓ Quality management systems under development - FDA compliance infrastructure
• FDA regulatory strategy defined with experienced counsel
• Quality systems implementation in progress - Design controls and risk management
• Clinical evidence package supporting market authorization
• Preparing for regulatory submission - Documentation and testing protocols
• Post-market surveillance planning underway
• Initial deployment through Florida rehabilitation networks
• Regional expansion strategy developed - Phased geographic rollout
• Reimbursement pathway analysis complete - Medicare coverage strategy
• Distribution partnerships in development - DME and specialty channels
• Real-world evidence generation planned - Supporting payor coverage
Our regulatory and commercialization strategy leverages extensive clinical validation to accelerate market entry while building sustainable reimbursement pathways. Focus on milestone achievement and evidence generation positions evergait™ for successful scaling and strategic partnerships.
Comprehensive research program with peer-reviewed publications demonstrating consistent therapeutic benefits for stroke survivors. Our evidence base continues to grow through ongoing clinical validation and real-world studies.
8 peer-reviewed publications in leading journals demonstrating consistent clinical efficacy and sustained therapeutic effects.
View PublicationsProven efficacy in multiple clinical trials with sustained benefits in gait speed, symmetry, and fall reduction.
Research DocumentationContinued clinical validation through IRB-approved research protocols expanding evidence base.
Learn MoreWorld-class research foundation meets proven commercial execution. Deep technical expertise combined with medical device go-to-market experience.


Regulatory Counsel: Working with experienced FDA regulatory attorneys and reimbursement specialists for strategic guidance on market pathway and Medicare coverage development.
Manufacturing: ISO 13485-certified partner selection underway for scalable production. Quality management system implementation in progress.
R&D Collaboration: Ongoing collaboration with USF REED Lab including graduate student researchers and undergraduate engineering teams supporting technology development and clinical validation.
evergait™ is building a capital-efficient path to market through a strategic combination of non-dilutive funding and strategic partnerships. Our approach prioritizes patient access and sustainable growth while building long-term value for stakeholders.
University Partnership: Licensed technology from University of South Florida with extensive research foundation
Seed Funding: BRAG Innovation Award recipient supporting initial development milestones
Federal Research Grants: Pursuing SBIR/STTR programs to support clinical validation and product development
Strategic Partnerships: Healthcare provider collaborations for pilot deployments and clinical data
Industry Collaborations: Working with medical device manufacturers and rehabilitation technology leaders
Our funding strategy emphasizes:
• Non-dilutive capital first to maximize founder ownership and exit value
• Clinical evidence generation to support regulatory pathway and reimbursement
• Strategic partnerships that provide both capital and market access
• Capital efficiency through leveraged university resources and grant funding
Our capital-efficient approach allows us to focus on what matters most: delivering clinical-grade gait rehabilitation technology to patients who need it. By prioritizing non-dilutive funding and strategic partnerships, we can build sustainable value while maintaining the flexibility to pursue optimal growth pathways.
Whether you're a healthcare provider interested in learning more, a researcher exploring collaboration, or a potential partner, we'd like to hear from you.
We welcome inquiries from healthcare providers, researchers, and potential collaborators interested in learning more about evergait™ technology.
Founder & Chief Technology Officer
Email: [email protected]
USF Email: [email protected]
Technical partnerships, research collaborations
Chief Executive Officer | USF Executive in Residence
Email: [email protected]
USF Email: [email protected]
Business inquiries, strategic partnerships
evergait™ LLC
Florida Registered LLC
4304 Carrollwood Village Drive
Tampa, FL 33618
Website: www.evergait.com
CAUTION: The evergait™ Go is an investigational medical device limited by Federal (USA) law to investigational use.
It is not cleared, approved, or authorized by FDA for commercial distribution.
All clinical studies conducted under IRB approval with informed consent. Information on this website is provided for
research collaboration, strategic partnership, and investor due diligence purposes only. Not promotional material for medical device.
No Commercial Availability: Device not available for prescription, purchase, or clinical use outside IRB-approved research protocols.
Commercial availability entirely dependent on FDA regulatory determination expected Q2 2026.